How To Sign Up For Covid Vaccine Trial Johnson And Johnson
Everyone who participates will follow up with a doctor. However it is important to understand that certain patients with allergies and some women under 50 according to the CDCs guidance on the JJ Vaccine.

Coronavirus South Africa To Use Johnson Johnson Vaccine Instead Of Astrazeneca
Janssen COVID-19 Vaccine Johnson Johnson.
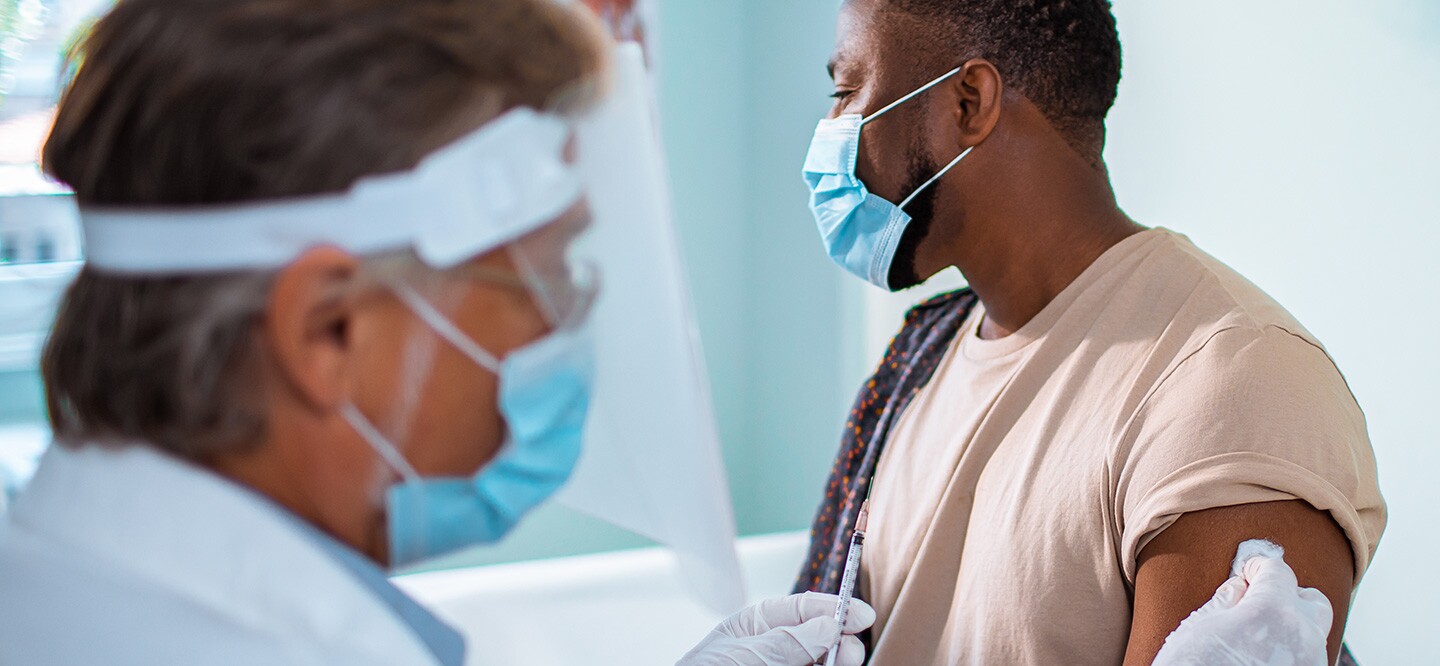
How to sign up for covid vaccine trial johnson and johnson. Johnson Johnson has begun dosing up to 60000 volunteers in a study of its Covid-19 vaccine marking the first big US. At AFC Urgent Care Clearwater healthy patients that want a single shot vaccination can opt to have the Johnson Johnson JJ COVID-19 vaccine. Appointments are encouraged and walk-ups are welcome.
The clinical trial is being conducted to evaluate the efficiency and safety of the Johnson and Johnson COVID-19 vaccine. Johnson Johnson expects to enroll up to 60000 people in multinational Phase 3 trials for its COVID-19 vaccine candidate. Trials for the single-dose vaccine will include up.
Johnson Johnsons Covid-19 vaccine candidate begins Phase 3 trials in the United States today. CINCINNATI Free COVID-19 vaccines will be available at the National Underground Railroad Freedom Center from noon to 3 pm. Trial of an inoculation that may work after just one shot.
Effective April 23 2021 CDC and FDA recommend that use of the Janssen COVID-19 Vaccine resume in the United States. Food and Drug Administration FDA but have been authorized for emergency use by the FDA under an Emergency Use Authorization EUA to prevent Coronavirus Disease 2019 COVID-19 for use in individuals 16 and older for Pfizer and 18 years of age and older for Moderna and Johnson Johnson. Unlike other possible vaccines JJs candidate only requires a.
COVID-19 Vaccine Waitlist sign up here The Houston Health Departments COVID-19 vaccine waitlists are open to people age 65 and older and people age 16 and older with chronic health conditions. Johnson Johnsons single-shot coronavirus vaccine protected against symptomatic and asymptomatic infection and prevented hospitalization and death in. In the case of the Johnson Johnson COVID-19 vaccine people with certain underlying medical conditions may still be able to participate in the ENSEMBLE studies if their signs and symptoms are stable and well-controlled.
Get the latest schedule of vaccination events. Its been added to the arsenal of vaccines approved for. Pulmonologist Njira Lugogo MD is the principal.
However women younger than 50 years old especially should be made aware of a rare risk of blood clots with low platelets following vaccination and the availability of other COVID-19 vaccines where this risk has not been observed. The newest vaccine available in the United States is the Johnson Johnson COVID-19 vaccine. Covid-19 vaccine trials from AstraZeneca Johnson Johnson to restart.
T wo major studies of vaccines against Covid-19 both paused because of. Johnson Johnson is one of many companies hard at work on an investigational COVID-19 vaccine candidate which includes recruiting up to 60000 adult participants 18 and older and of all genders and racial and ethnic groups around the world for a Phase 3 clinical trial known as ENSEMBLE. The Pfizer Moderna and Johnson Johnson vaccines have not been approved or licensed by the US.
Trials showed that the Johnson Johnson vaccine was more than 66 effective when it came to preventing illness in people who received it. For example the ENSEMBLE trial protocol says that people with certain autoimmune conditions may be eligible to enroll at.
Three Stories Of What It S Like To Participate In A Clinical Trial Johnson Johnson

Johnson Johnson Covid 19 Vaccine Study Paused Due To Illness

Answers To Questions About Johnson Johnson S Covid 19 Vaccine Shots Health News Npr
Early Data Shows Promise For Johnson Johnson S Covid 19 Vaccine

How The New One Dose Covid 19 Vaccine Compares To Pfizer And Moderna Daily News

What You Should Know About Johnson Johnson S Covid 19 Vaccine

Johnson Johnson Will Run Covid Vaccine Trials On Infants
Johnson Johnson S Covid 19 Vaccine Safely Elicits An Immune Response

The 5 Stages Of Covid 19 Vaccine Development What You Need To Know About How A Clinical Trial Works Johnson Johnson
Johnson Johnson Is Starting A Trial To Vaccinate Adolescents

Massachusetts Explores The Advantages And Potential Challenges Of The Johnson Johnson Vaccine Commonhealth
The Johnson Johnson Vaccine Candidate For Covid 19 Explained

Volunteers Needed For Johnson Johnson Covid Vaccine Trial

Single Shot Covid 19 Vaccine Trial Likely In India Soon Johnson Johnson In Touch With Centre
I M In The Johnson Johnson Covid 19 Vaccine Trial Here S What It S Like

Jj Vaccine Single Shot Johnson Johnson Covid Vaccine 66 Percent Effective Against Moderate And Severe Illness The Washington Post
About Our Ensemble Studies Johnson Johnson
J J Covid Vaccine Distribution In Poor Black Communities Raises Race Questions

Covid 19 Vaccine Trials 9 Things You Should Know Hackensack Meridian Health
Post a Comment for "How To Sign Up For Covid Vaccine Trial Johnson And Johnson"